Fiber Photometry


Fiber photometry (or “FP”) is a widely used technique in neuroscience to optically measure neural activity in live animals, in combination with genetically encoded fluorescent indicators. This method is particularly valuable for studying brain function and behavior because it allows researchers to monitor specific populations of neurons in real time. We are using this platform to understand how multiple neuromodulators guide flexible decision-making, such as foraging for reward in a dynamic environment. Overall, fiber photometry is a powerful tool for understanding neural circuits' function and dynamics in behaving animals, providing insights into how specific brain regions, cell types, and neuromodulators/transmitters contribute to complex cognitive behaviors.
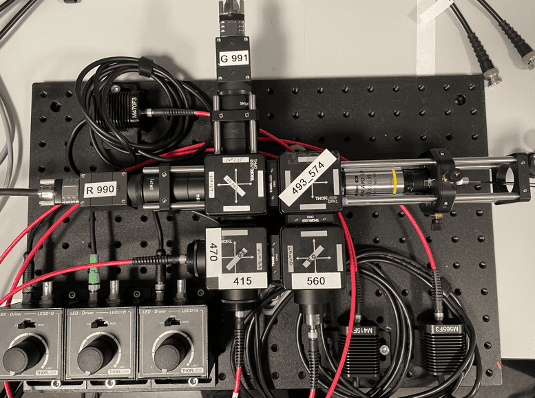
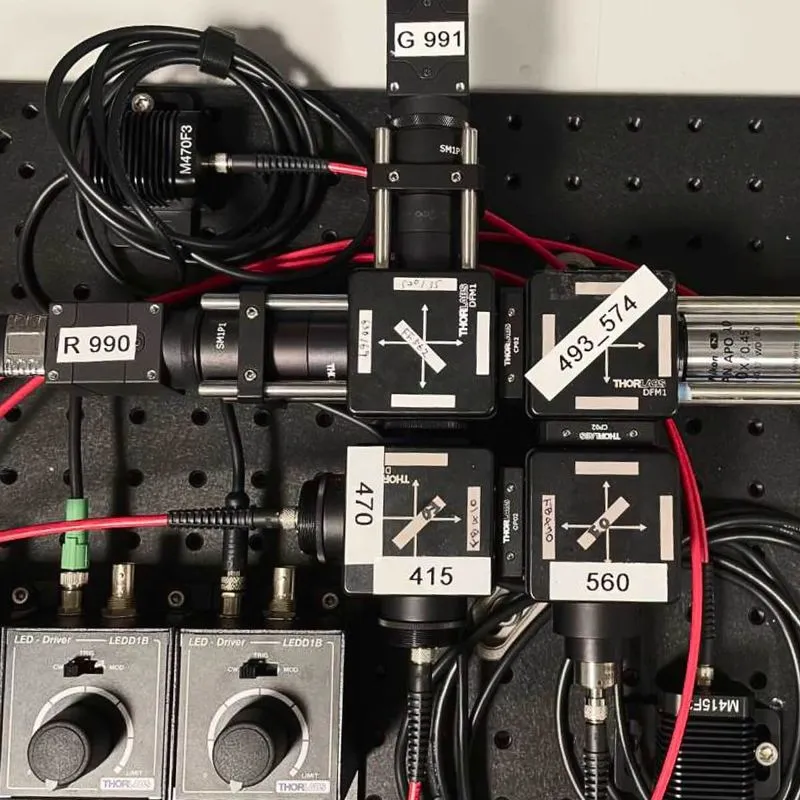
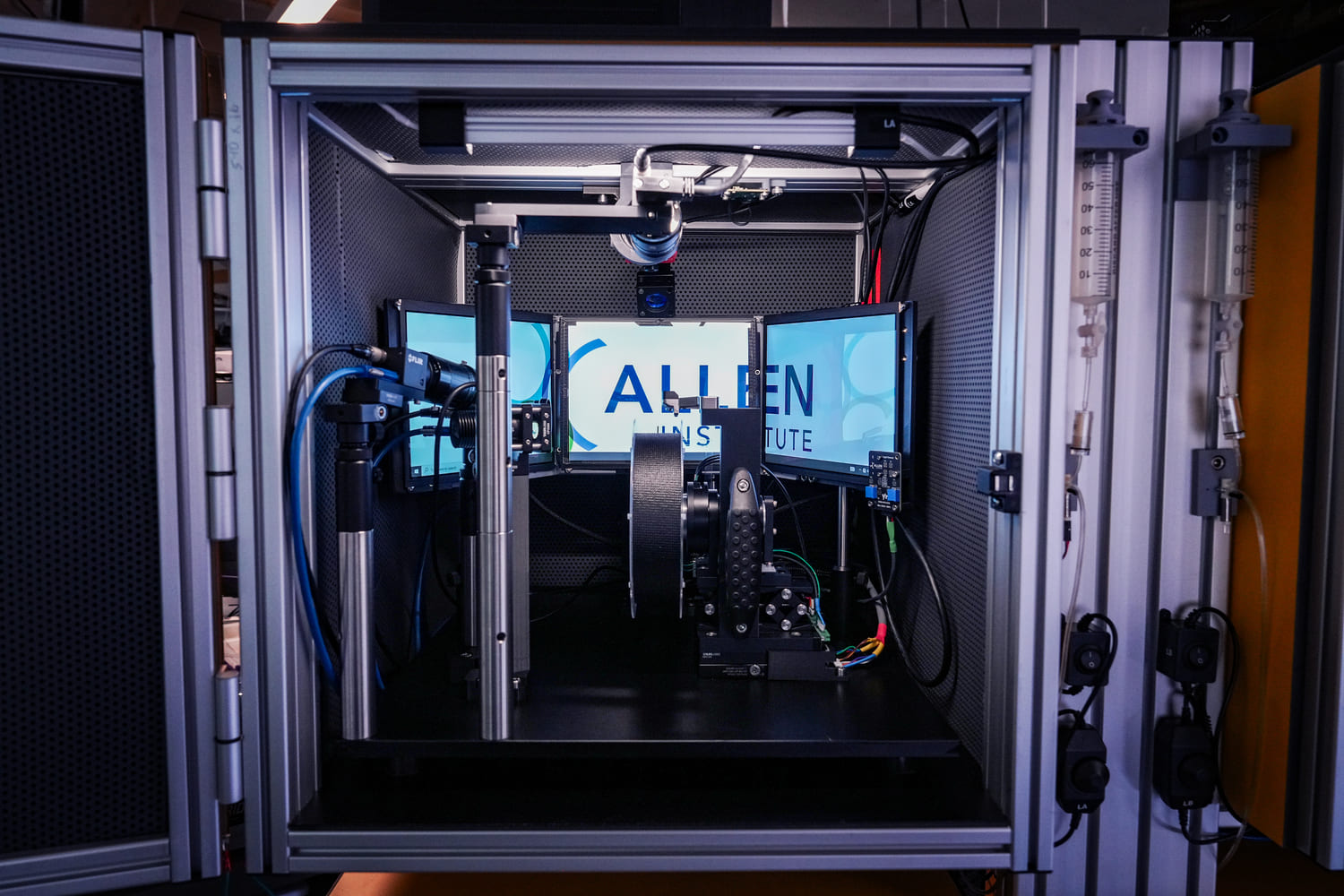
We need scalable photometry setups that, in combination with behavioral training rigs, can be used to chronically record optical signals from behaving mice during daily training. To address this, we modified an existing design (Frame-projected Independent Photometry; Kim et al., 2016), relying only on inexpensive, commercially available, off-the-shelf components. To complement the hardware, we have developed data acquisition software in Bonsai, an open-source software platform. Both the hardware and software are modular, and thus, excitation light sources and optical filter settings can be quickly changed, and the system can be combined with virtually any behavioral apparatus and with other recording devices. Our hardware designs and software are freely available through protocols.io.
In AIND, we use this system in combination with multiple behavioral paradigms, in particular, to measure neuromodulator dynamics in mice engaging the dynamic foraging task. Furthermore, by integrating this system into the large-scale electrophysiology platform we perform simultaneous optical and electrophysiological recordings in the same brain areas.

To allow the large community of users to better design and interpret optical measurements in behavioral experiments, we will perform rigorous and systematic in vivo benchmarking of indicators, particularly genetically encoded neuromodulator indicators. We will develop and assess benchmarking protocols and standards using optogenetic stimulation calibrated to behavior-associated signals. For each indicator class, we will select appropriate cell types, brain regions, behavioral calibrations, and stimulation patterns to enable fair comparisons between indicator variants. Resultant data will be rapidly shared as a growing online database.
The Surgery team offers a variety of aseptic rodent surgical procedures ranging from stereotaxic injections to headpost implantation and cranial windowing.
The Neuropixels platform uses pioneering technology for highly reproducible, targeted, brain-wide, cell-type-specific electrophysiology to record neural activity from defined neuron types across the brain.
The Molecular Anatomy platform combines innovative histology, imaging, and analysis techniques to map the morphology and molecular identity of neuron types across the whole brain.
The Fiber Photometry platform enables optical measurement of neural activity in live animals to study neural circuits' function and dynamics in behaving animals.
The Behavior platform uses advanced technology to implement a standardized, modular, multi-task virtual reality gymnasium for mice, with the goal to study brain function across different behaviors at scale.