.jpg)
Modified Frame-projected Independent Fiber Photometry (FIP) System_Hardware
This is a step-by-step protocol to build a modified FIP (Frame-projected Independent Fiber Photometry) system. FIP was first implemented and reported by Kim et al. (2016). Their protocol is available here.
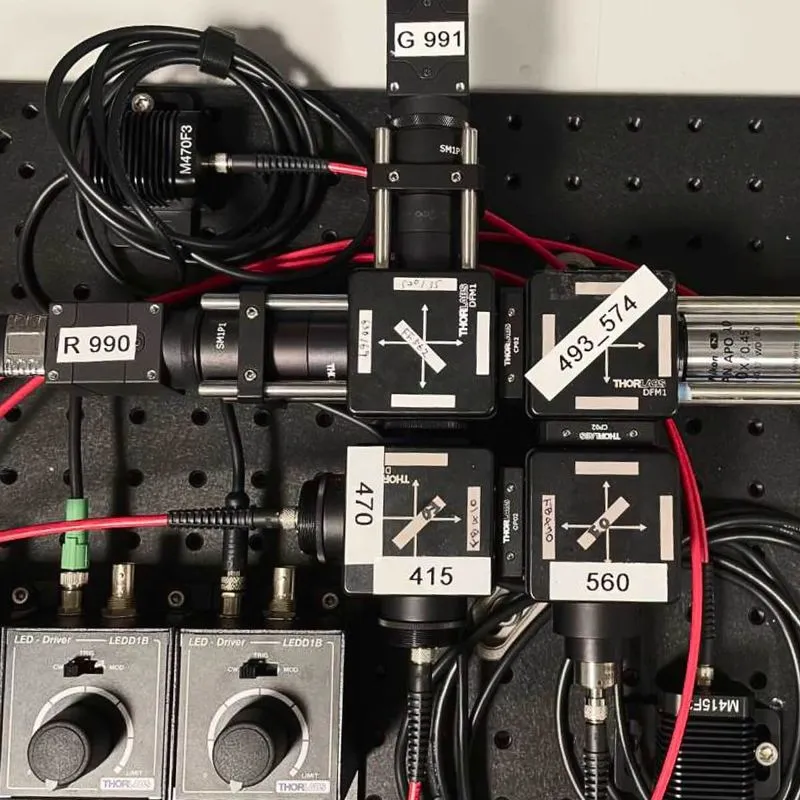
We have modified the previous design so that it can be built primarily with Thorlabs products (some products from other manufacturers are still required), which are off-the-shelf and easy to obtain with a reasonable lead time. The system described in this protocol is designed to record signals from 1) GFP-based sensors, 2) mApple-based sensors, and 3) isosbestic signals from up to 4-9 sites depending on fiber bundle selection (Fig. 1 and 2). When applied to measurements of other fluorescent protein-based sensors or spectrally shifted sensors, the selection of optical filters and/or excitation light sources should be modified accordingly. Also, the selection of the fiber patch-cable should be based on the number of simultaneously recorded sites and the diameter/NA of the fiber implants.